A Recap of 2022’s Orphan Drug Approvals

In the US, a rare disease is defined as one that affects less than 200,000 people. While these specific diseases are considered rare, living with a rare disease is not. In the US alone, over 30 million people are living with 7,000 different types of rare diseases.1
A 40-year legacy
The FDA established the Orphan Drug Act in 1983 to help counter the numerous challenges facing rare disease drug developers. Year over year, the industry has been spurred on by the advantages that this Act provides, including incentives such as:
- waiving the multimillion-dollar Prescription Drug User Fee
- providing tax credits for clinical testing
- providing 7-year exclusivity to the drug post-approval
- drug development grants available through the Orphan Product Grants Program
- eligibility for the new RDEA program for endpoint development
The program has seen much success. Over 6,000 Orphan Drug Designations (ODDs) have been granted since its inception, and, as seen by the graph below, the number of ODDs continues to increase.2
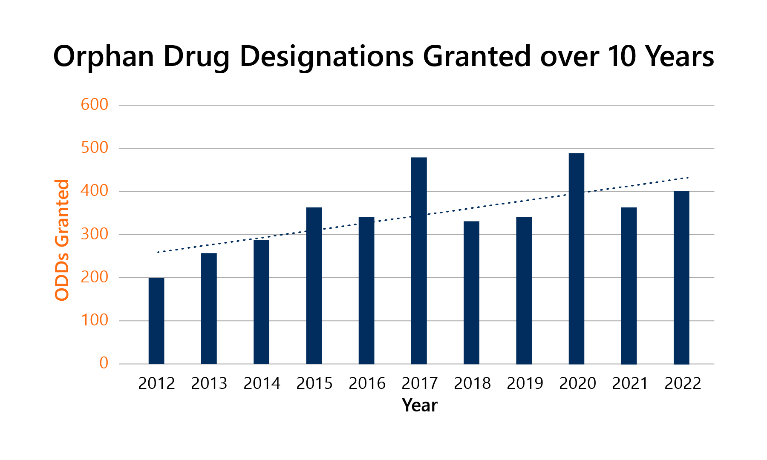
2022 in review
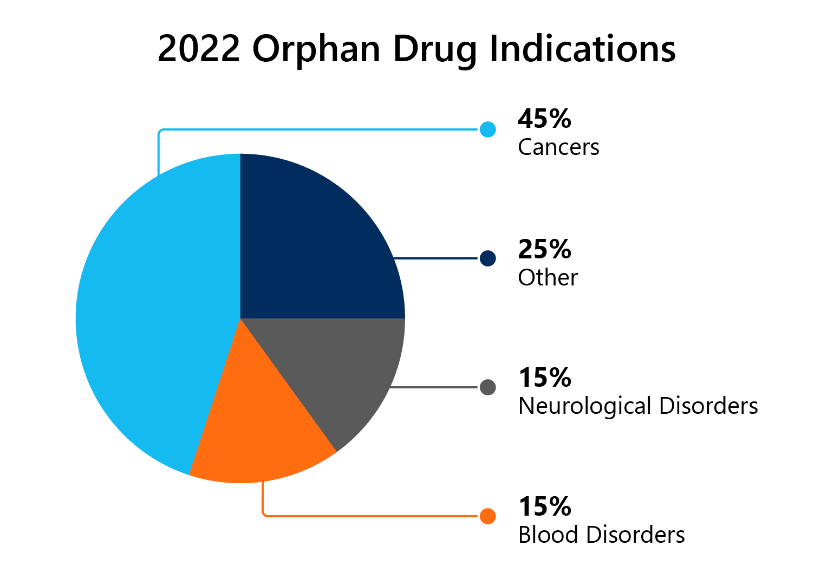
Last year 54% of all novel drugs approved in 2022 were granted ODD. That is 20 out of a total 37.
9 of the 20 designations (45%) were for cancers, 3 (15%) for nervous system disorders, and 3 (15%) for blood disorders. This can be seen in the pie chart below. 3
Because of the serious nature of rare diseases, 85% of these drugs also qualified for additional expedited development and review pathways, with 65% receiving two or more additional expedited designations. These drugs have other notable categorizations outlined below. 3
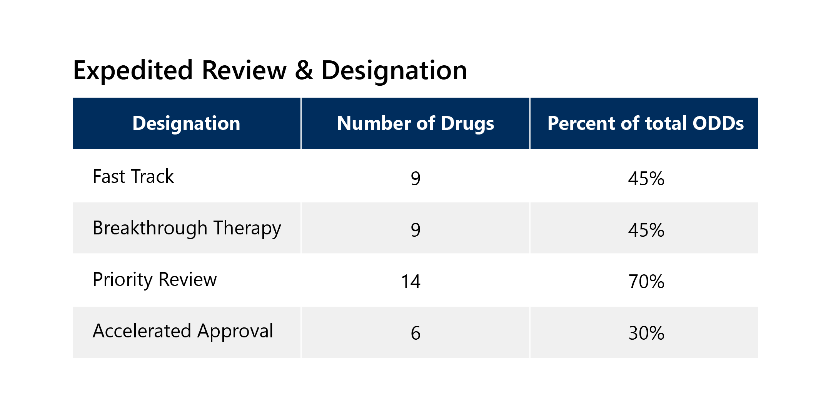
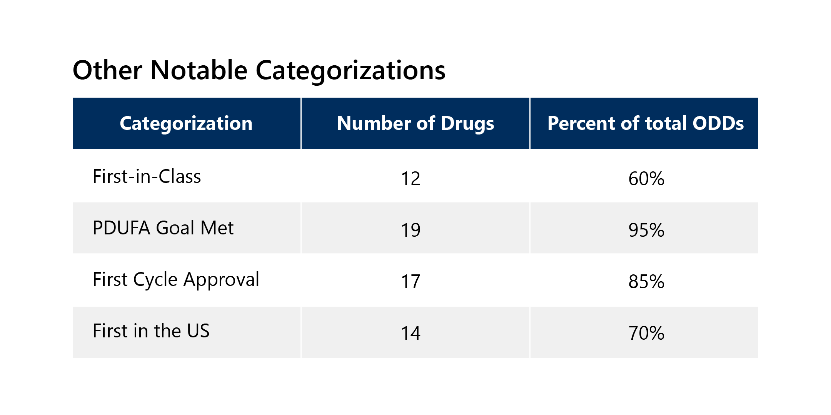
List of Novel Drugs with ODD Approved in 2022
Oncology
Elahere (mirvetuximab soravtansine-gynx) – First-in-Class – treats recurrent ovarian cancer resistant to platinum therapy.
Imjudo (tremelimumab-actl) – treats unresectable hepatocellular carcinoma, the most common type of liver cancer.
Kimmtrak (tebentafusp-tebn) – First-in-Class – treats metastatic or unresectable uveal melanoma.
Krazati (adagrasib) – treats patients with advanced, KRAS-mutated colorectal cancer.
Lunsumio (mosunetuzumab-axgb) – First-in-Class – treats adults with relapsed or refractory follicular lymphoma.
Lytgobi (futibatinib) – treats intrahepatic cholangiocarcinoma harboring FGFR2 gene fusions or other gene rearrangements.
Opdualag (nivolumab and relatlimab-rmbw) – First-in-Class – treats those aged 12 years or older with metastatic or unresectable melanoma.
Tecvayli (teclistamab-cqyv) – First-in-Class – treats adults with relapsed and refractory multiple myeloma who have received at least four prior therapies.
Rezlidhia (olutasidenib) – treats relapsed/refractory acute myeloid leukemia with IDH1 mutation.
Neurology
Amvuttra (vutrisiran) – treats polyneuropathy in adults with hereditary transthyretin-mediated amyloidosis.
Relyvrio (taurusodiol and sodium phenylbutyrate) – treats amyotrophic lateral sclerosis (ALS).
Ztalmy (ganaxolone) – First-in-Class – treats seizures associated with cyclin-dependent kinase-like 5 deficiency disorder (CDD). This is the first treatment for seizures associated with CDD and the first treatment specifically for CDD.
Blood Disorders
Enjaymo (sutimlimab-jome) – First-in-Class – decreases the need for red blood cell transfusion due to hemolysis in adults with cold agglutinin disease.
Pyrukynd (mitapivat) – First-in-Class – treats hemolytic anemia in adults with pyruvate kinase deficiency.
Vonjo (pacritinib) – treats adults with a rare bone marrow disorder, myelofibrosis, and who have low platelet (blood clotting cells) levels.
Other
Camzyos (mavacamten) – Cardiology – First in Class – treats adults with symptomatic obstructive hypertrophic cardiomyopathy.
NexoBrid (anacaulase-bcdb) – Dermatology – aids in the removal of dead skin caused by burns (eschar) in adults with deep partial & full thickness thermal burns.
Spevigo (spesolimab-sbzo) – Dermatology – First-in-Class – treats flares in patients with generalized pustular psoriasis.
Terlivaz (terlipressin) – Nephrology – First-in-Class – improves kidney function in adults with hepatorenal syndrome.
Xenpozyme (olipudase alfa-rpcp) – First-in-Class – treats non-central nervous system manifestations of acid sphingomyelinase deficiency (ASMD).
The Future of Rare Disease Drug Development
Ongoing innovation in the rare disease space continues to lead to the development of novel therapies and targeted treatments for rare disease patients.
The FDA, in partnership with various community organizations, drug development Sponsors, and other federal agencies, has begun to compile community research and development tools to advance breakthroughs and explore new technologies through its precisionFDA platform. This platform allows for global consolidation and sharing of research to enable the development of treatments rare disease through a targeted, personalized medicine approach. 4
With the FDA’s focus on developing individualized therapies, we expect to see many approvals and orphan drug designations targeted at the most vulnerable patient populations.
If you have a question about orphan drug designations or rare disease research and development, please email info@mmsholdings.com to be connected to an expert.
By: Aaron Pyle, Regulatory Affairs Associate and Ritchie Patton, Global Regulatory Affairs Manager
References
Suggested For You

perspectives
August 24th, 2023
“D” is for Discipline: Everything You Need to Know About FDA's Type D Meetings

perspectives
August 2nd, 2023
Single-Arm Trials and Drug Approval in the European Union (EU)

perspectives
March 16th, 2023
New FDA Policies and Procedures for Products Submitted Under Accelerated Pathways : Understanding CDER MAPP 5015.13

perspectives
March 7th, 2023
SENDing Successful Nonclinical Submissions through Validation, Review, and Beyond

perspectives
February 2nd, 2023
FDA Updates IND Expanded Access Guidance for Industry

perspectives
November 30th, 2022
Updates to FDA Meetings under PDUFA VII

perspectives
November 29th, 2022
CMC and Quality Enhancements Under PDUFA VII Explained

perspectives
November 15th, 2022
What is the Rare Disease Endpoint Advancement Pilot Program and What Does it Mean for Rare Disease Research

perspectives
November 15th, 2022
Split Real-Time Application Review (STAR) Pilot Program Explained: PDUFA VII Updates

perspectives
October 26th, 2022
Prescription Drug User Fee Act (PDUFA) VII Updates Explained

perspectives
August 22nd, 2022
How to Achieve Compliance for Feasibility Studies with Digital Health Technologies

perspectives
January 24th, 2022
FDA’s Recent Benefit Risk Assessment Guidance Explained: What It Means for Sponsors