Building a robust clinical data science process
During PhUSE US Connect 2018 – the clinical data science conference – in Raleigh, North Carolina, MMS data science experts presented a paper and corresponding poster, titled Big Data Paradox: Development of a Unique and Repeatable Analytical Process while Implementing a Robust Data Science Solution.
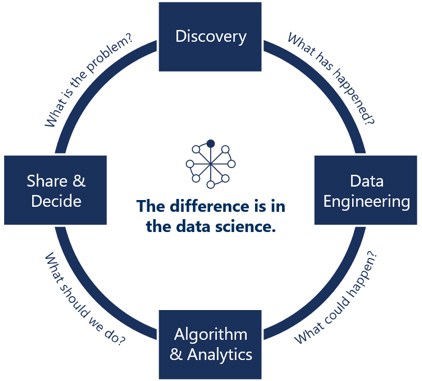
Security and auditing: Data landing, storage, cleaning, standardization, analysis, prediction, and security
Authored by MMS experts Chris Hurley, Aditya Gadiko, Srinivas Mukkala, and Kulin Mehta, the paper’s abstract is as follows:
Who could have imagined that a few simple ASCII datasets would pose such a huge “big data” challenge? Therein lies the paradox, while the data is simple and there are relatively few variables to analyze, the volume is huge and presents a great processing challenge. This poster demonstrates a repeatable “big data” solution that utilizes FAERS data, provided quarterly by the FDA, to provide the answers to post-marketing safety surveillance questions. The process, techniques, and tools used to handle and analyze such massive amounts of data, in a clinical setting, are demonstrated here, creatively enabling us to cut the Gordian knot and overcome a seemingly impossible challenge.
To learn the robust clinical data science process behind this abstract, read the full paper here.
Suggested For You

perspectives
June 6th, 2024
Datacise and Diversity in Patient Enrollment: Combining Geospatial and Demographic Data to Aid Site Selection

perspectives
April 29th, 2024
Validation of Clinical Dashboards for Decision Making

perspectives
December 14th, 2023
Data Provenance in Real World Evidence Studies, Explained!

perspectives
September 8th, 2023
FDA and the Real-World: Key Changes from Draft to Final Guidance on RWD and RWE

perspectives
November 30th, 2022
Exploring the use of Real-World Evidence in Regulatory Decision Making Under PDUFA VII

perspectives
August 16th, 2022
Natural Language Processing in Healthcare: The Pros, Cons, and Potential Impact

perspectives
May 31st, 2022
Attending the 2022 PHUSE US Connect: A Data Scientist’s Experience

perspectives
June 10th, 2021
How to Use Machine Learning in Clinical Research

perspectives
July 10th, 2020
Why COVID-19 testing devices require a high level of specificity

perspectives
February 7th, 2020
The New Roaring 20s: 12 Experts Provide Pharmaceutical Insights for the Decade Ahead

perspectives
August 22nd, 2019
Using Real World Data in Drug Development: Five Questions with Vijay Ivaturi, PhD

perspectives
July 18th, 2019
Using Real World Data in Pharmaceuticals: A Conversation with Chris Hurley